Vaccination rollout is not your typical supply chain challenge…and cold chain monitoring is a must
Thankfully this year, a number of pharmaceutical companies have released Covid-19 vaccines, giving the world new hope of recovery and renewal after the initial crisis. The role of vaccines in controlling the pandemic is fixed in the public consciousness, with efficacy rates and clinical trial processes almost dinnertime conversation.
Now, the shift in focus is toward the widescale vaccine supply chain and administration planning and processes that are necessary, putting governments, transportation companies and healthcare providers center stage.
New complexities
The Covid-19 global vaccination journey has just begun. As vaccines move from clinical trial to production, different stakeholders across the ecosystem – from national and local governments, to distributors and logistics companies, to vaccine administration entities such as community health organizations, healthcare providers, retail sites, employers and more – must all learn and apply new processes and systems.
Vaccine production and delivery bring significant challenges, such as production delays, the demands of maintaining an ultra-cold supply chain, the need to manage prioritization of populations to receive vaccinations, the complexities of scheduling thousands of appointments, ensuring the quality of vaccines, tracking recipients for follow-up, and mobilizing citizens and medical staff.
Each vaccine incorporates its own highly specific technology, particular dosage requirements and percentages of efficacy; each requires different processes to comply with precise temperature stability parameters.[1] In addition, each country and community has different approaches and entities responsible for distributing and administering vaccinations for different populations.[2]
Managing the supply chain
To ensure that opportunities to vaccinate are not missed, the vaccine supply chain must achieve the six ‘rights’ of supply chain management:[3]
- Right product
- Right quantity
- Right condition
- Right place
- Right time
- Right cost
Temperature monitoring is a must at each step of the process: every country, every state, every box, every truck, every refrigerator, every cooler, every vaccine.[4]
And that’s focusing only on the vaccine itself: we must also consider the supply of all vials, syringes and stabilizing agents needed for the end-to-end vaccine administration process.
Temperature monitoring is a must at each step of the process: every country, every state, every box, every truck, every refrigerator, every cooler, every vaccine.
Once the vaccine is received, administering organizations face the challenge of communicating to their communities when, where and how vaccines will be administered. They have to respond rapidly to situations that limit supply, such as recent unprecedented winter weather in the southern US; and they need to change the process for those awaiting their first vaccine from those needing their second.
Digital enablement for intelligent supply chains
Technology can help at each step of the way to create an intelligent supply chain. For example, the opportunity to use wireless data loggers in combination with digital automation and monitoring capabilities is critical to ensure success of such complex logistics projects on a global scale. Digital loggers monitor temperatures from -200°C to 800°C, as well as differential pressure, humidity and carbon dioxide across the supply chain (fridge, freezer, transport and storage).
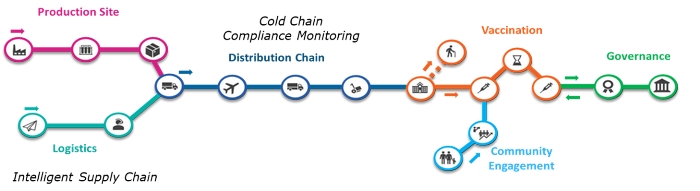
This type of solution could offer a fully automated service that optimizes resources and eliminates the risk of error and breach of compliance, including monitoring and maintaining transportation & shipment compliance, tailored to customers’ needs with value throughout the process. Benefits include complete transparency of temperature monitoring, plus built-in end-to-end traceability with location tracking. Quality systems are maintained, with auto-calibration systems and alerts/notifications if temperature thresholds are broken.
Recovery from Covid-19 must be supported by advanced technologies and data, which have a critical role to play as vaccinations programs are rolled out and embedded. From identifying the right patient, to planning vaccination programs in line with priorities, to ensuring that the cold chain has not been broken during transportation, and to monitoring and tracking post-vaccination, technologies will underpin the journey to a healthier post-pandemic world.
[1] Tracking COVID-19 vaccines and therapeutics | McKinsey
[2] Covid-19 vaccine tracker (ft.com)
[3] WHO | Immunization supply chain and logistics
[4] The COVID-19 Vaccine US Supply Chain | Supply Chain Management Center (ufl.edu)
By Dr. Neeta P. Bhatia, Senior Consultant, Healthcare Practice
Posted on May 18